ScienceSampler
Myosin VI is an unusual motor
Myosin VI Stabilizes an Actin Network during Drosophila Spermatid Individualization
Tatsuhiko Noguchi, Marta Lenartowska, and Kathryn G. MillerMol. Biol. Cell 2006 17: 2559-2571
Similar to other myosins, myosin VI contains an ATP-dependent motor domain, a coiled-coil domain, and a globular tail domain. However, myosin VI moves towards the pointed end as opposed to the barbed end of an actin filament. Previous work has implicated myosin VI as a motor for endosomal movement due localization studies and in vitro assays showing the ability for myosin VI to form dimers and move processively. However, myosin VI can also act as an actin dependent molecular crosslinker. When expressed in baculovirus, a majority of myosin VI is monomeric and shows no processive movement.
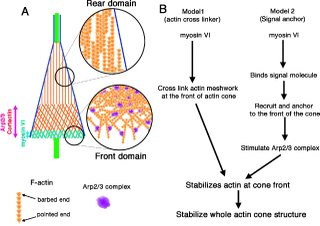
This paper sheds light on the role of myosin VI in vivo during spermatogenesis in drosophila, specifically in the actin cone. The deletion of myosin VI decreases both the relative amount and density of F-actin as opposed to wild-type in actin cones, while overexpression of myosin VI has the opposite effect. These data combined with the effect of myosin VI deletion on actin cones by EM, and the persistence of GFP-myosin VI after FRAP, illustrates myosin VI's role as a crosslinker. The above cartoon shows, a role for myosin VI stabilization of the branched actin meshwork at the front of actin cones. No data obtained from this paper implicates myosin VI as a cargo transporter.
Additionally, the structure of the actin cone is unique as the filaments in the cone are oriented with their barbed ends facing away from the direction of movement. This mechanism is opposite of that found in Listeria comet tails and lamellipodia where barbed ends face toward the direction of movement. This difference causes the authors to speculate on the actin polymerization mechanism which is also depicted in the cartoon.

0 Comments:
Post a Comment
<< Home