Actin differentiation through Arginylation
In cells, actin polymers dictate cell morphology.
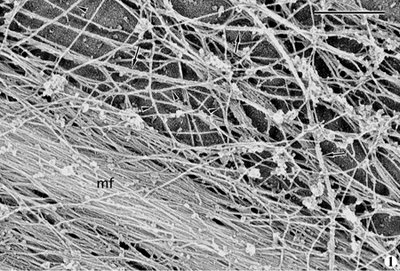 Actin filaments can adopt several conformations, they can be bundled into large microfilaments (often called stress fibers; here "mf" - electron micrograph taken from the Borisy lab webpage) or arranged in a meshwork (as seen in the second electron micrograph). One actin isomer (Gamma-actin) predominates in the stress fibers that are present in the cell body, while another (beta actin) predominates in the actin meshwork found in the leading edge of migrating cells.
Actin filaments can adopt several conformations, they can be bundled into large microfilaments (often called stress fibers; here "mf" - electron micrograph taken from the Borisy lab webpage) or arranged in a meshwork (as seen in the second electron micrograph). One actin isomer (Gamma-actin) predominates in the stress fibers that are present in the cell body, while another (beta actin) predominates in the actin meshwork found in the leading edge of migrating cells. 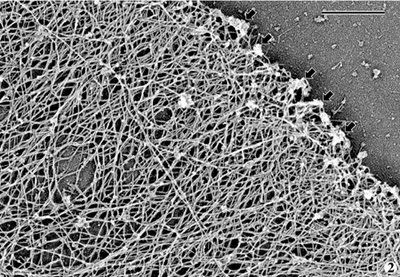 In fact, the generation of this meshwork right at the tip of the cell (see arrows in the first figure) is thought to push the cell membrane forward. This leading edge polymerization provides the major force for membrane spreading and plays an important role in cellular locomotion.
In fact, the generation of this meshwork right at the tip of the cell (see arrows in the first figure) is thought to push the cell membrane forward. This leading edge polymerization provides the major force for membrane spreading and plays an important role in cellular locomotion. So if gamma-actin makes up microfilaments, and beta-actin makes up the meshwork, how do the two isoforms differ? How is the differential distribution explained? Beta-actin mRNA is concentrated at the leading edge and is thought to be synthesized there, but most researchers thought that there must be more to the story than that. Gamma-actin and beta-actin differ by only 4 out of 100 residues, and both genes are EXTREMELY well conserved across different species (100% conserved at the amino acid level between birds and humans).
A couple of weeks ago a great paper came out in Science about actin arginylation that cleared things up a bit.
For many years it had been noted that tubulin, the building block of the microtubule cytoskeleton, undergoes many post-translational modifications (see my post on this topic). These modifications help to differentiate microtubules that are aligned along the cell's main axis from the bulk microtubules that are present through out the cell. Now a group from U Penn who were studying arginine tRNA protein transferase (Ate1), discovered that actin gets post-translationally modified too. Bottom line - Ate1 adds arginine onto beta-actin's N-terminal glutamate side chain.
 This modification (similar to what was seen in tubulin & microtubules) aids in differentiating a part of the actin network from bulk actin filaments.
This modification (similar to what was seen in tubulin & microtubules) aids in differentiating a part of the actin network from bulk actin filaments. Now comes the new stuff ...
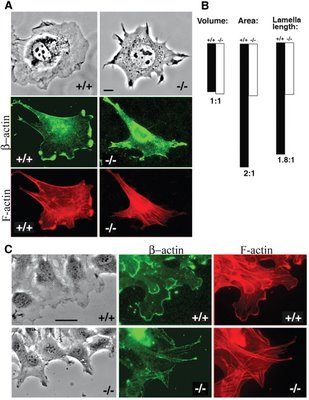
In the recent Science paper, the authors report that beta-actin, but not gamma-actin, gets arginylated by Ate1. This is supported by the finding that beta-actin arginylation is abolished in cells lacking Ate1. Aha! Perhaps arginine may give beta-actin the required properties to form meshworks. In agreement with this idea, Ate1 -/- cells have reduced levels of actin meshwork, and show defects in cell spreading and cell migration (see the figure, right). So then the big question is: how does arginylation affect beta-actin?
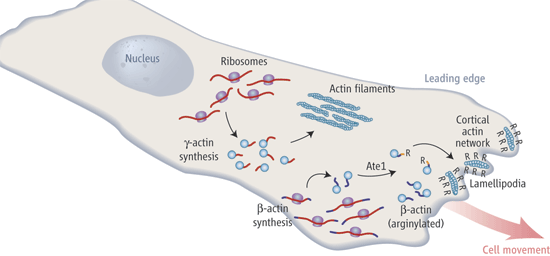
(here is a good schematic I ripped off of Chloe Bulinski's nice commentary in the same issue of Science).
The authors speculate that the extra positive charge from arginine may help prevent actin from bundling through electrostatic repulsion. Quite honestly I do not believe that the answer will be so simple. The actin meshwork and filaments are formed by separate mechanisms. The meshwork is formed by the polymerization of actin "branches" by the arp2/3 complex. Microfilaments are formed (probably) by formins which wrap around actin, forcing it into long fibers. These fibers are then bundled by the motor protein myosin. It is then likely that arginylation affects one of these actin-modeling processes.
In conclusion this paper offers a neat new concept for the cytoskeletal field.
Ref:
Marina Karakozova, Marina Kozak, Catherine C. L. Wong, Aaron O. Bailey, John R. Yates, III, Alexander Mogilner, Henry Zebroski, Anna Kashina
Arginylation of ß-Actin Regulates Actin Cytoskeleton and Cell Motility
Science (2006) 313:192-196

1 Comments:
Nicely described about the actin. Very good article.
Thanks
Joshua Thompson
Arginine Benefits
Post a Comment
<< Home