Something in the tubes
For my inaugural post in the blog, I wanted to share a paper that covers a topic that I am really excited about. It's something I became interested in as a graduate student hanging out in Gregg Gundersen's laboratory and hope to spend some time on now that I'm a post-doc. It gets at the fundamental difference between microtubules and the other cytoskeletal proteins (actin and intermediate filaments). Unlike the other two elements, microtubules (as their name implies) are indeed tubes. The protofilaments assemble in such a way as to create an inner core with a diameter of 16 nanometers (admitedly small but would support a globular protein somewhere in the range of 300 kiloDaltons). Although a great deal of work has gone into studying proteins that bind the outer surface of microtubules (motors, MAPs, plus-end tracking proteins, etc.), very few people are looking at the inside. A recent publication in the journal Nature may get a few more people thinking about it.
Haixin Sui and Kenneth Downing, working at the Lawrence Berkeley National Laboratory did a cryo-electron tomography (fancy high resolution imgaing) study of axonemal microtubules (the microtubules found in cilia and flagella). In these structures, a stereotypical doublet of microtubules forms as is shown in the picture here:

Upon careful examination of their images, they noticed densities in the lumen of the microtubule (the inner surface) that could not be accounted for by fitting the known crystal structure of tubulin to their images. As they analyzed the data along the length of the microtubules, they found that these densities exhibited periodicity consistent with the known distance between tubulin monomers. Through the analysis of thousands of images, they generated a 3D density map of the microtubule inner surface as shown here:
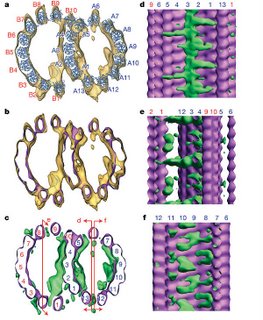
They suggest that the shape and size of one group of these proteins would identify them as Tektins, an intermediate filament-like family of proteins previously co-purified with axonemal microtubules. However, the identity of the other proteins in the microtubule lumen remains a mystery (although they suggest that the other proteins may be Sp77 and Sp83, proteins previously identified in sperm flagella).
Previous studies have suggested that there must be proteins on the inner lumen of microtubules. Indeed, acetylation of microtubules takes place on a lysine that is predicted to orient toward the lumenal surface. However, this is, to my knowledge, the most elegant description of microtubule lumenal proteins to date.
What are these proteins? Do they contribute to the structure of the axonemal microtubules? Are there similar proteins in the lumen of the interphase array of microtubules in other cells? These questions seem to be wide-open areas for future study.
Reference:
Sui, H. and Downing, KH. (2006) Molecular architecture of axonemal microtubule doublets revealed by cryo-electron tomography. Nature 442, 475-478.

4 Comments:
Hi TUBE,
welcome to sciencesampler blog!!!
Ha! Cool paper - I guess microtubules must breathe. Just to show that I know who micrTUBEules are ... actin sucks, right?
PS. I thought I was the ONLY ONE who regularly posted here! (In other words it's nice to see that someone has picked up the slack.)
Well, mad scientist, I haven't seen a single original post from you. ALL your post are from "the daily transcript". So I guess you should start writing some original stuff like everybody else, instead of copy-paste your posts...
Hmm. I thought that I was posting the original HERE but then some guy at ScienceBlogs always plagerizes my work ...
Post a Comment
<< Home