Something in the tubes, and Dynein!
A new paper from Dick McIntosh's group appeared in Science this week that is similar to the paper in Nature I highlighted last week. In fact, the title is almost identical (The Molecular Architecture of Axonemes Revealed by Cryoelectron Tomography vs. Molecular Architecture of Axonemal Doublets Revealed by Cryo-electron Tomography). However, in my opinion, the paper by the McIntosh group covers a great deal more ground than the paper by Downing's group. But, read them both and decide for yourself.
Flagella are composed of 9 microtubule doublets surrounding the central pair of single microtubules. Movement of flagella takes place as a result of pulling forces exerted by the microtubule-dependent motor, dynein. Nicastro et al. performed cryoelectron tomography of flagella from Chalmydomonas and sea urchin sperm and did 3D reconstruction of the entire network. The resolution level of their images of dynein provide insight not only into the localization of dynein relative to the microtubules, but also possible mechanisms of dynein activity as a result of the orientation of different parts of the dynein heavy chain. Summarizing models are shown below.
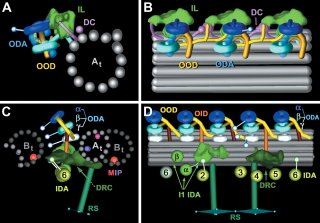
Of interest to me (you might have guessed) is that they also saw significant densities on the inner lumen of the microtubule doublets, which they call MIPs (Microtubule Inner Proteins). Three significant MIPs are observed in this study which all exhibit 8 or 16 nm periodicity along the MT lattice. They suggest that these unknown proteins may provide stability to the long-lived microtubules in the flagellum - just as taxol, which binds to the inner surface ot microtubules, also stabilizes microtubules. However, they make no strong statements as to the identity of these molecules.
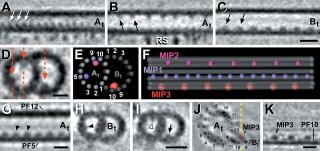
I find it interesting to compare the results from the two EM studies (if you compare them side by side, flip one of them 180 degrees because they present their data in opposite orientations). Although they both picked up similar densities in the A-tubule, the McIntosh group does not see the density suggested to be Tektins by the Downing group. Conversely, the prominent density in the B-tubule seen by the McIntosh group is not reported in the work from the Downing group. Instead, they see a density on the other side of the B-tubule. Oddly, neither group see the "ponticulus" identified by Vaughan et al. Since they both used sea urchin sperm for at least part of their work, why wouldn't they see exactly the same thing? Someone more familiar with cryo-EM methods may be able to show where the two preparations are different. If that is true, does it mean that they both have incomplete models and there is more inside the MT lumen than either suggest individually?
LINK:
Nicastro, D., Schwartz, C., Pierson, J., Gaudette, R., Porter, M.E., and McIntosh, R. 2006. The Molecular Architecture of Axonemes Revealed by Cryoelectron Tomography. Science 313: 944-948.

0 Comments:
Post a Comment
<< Home